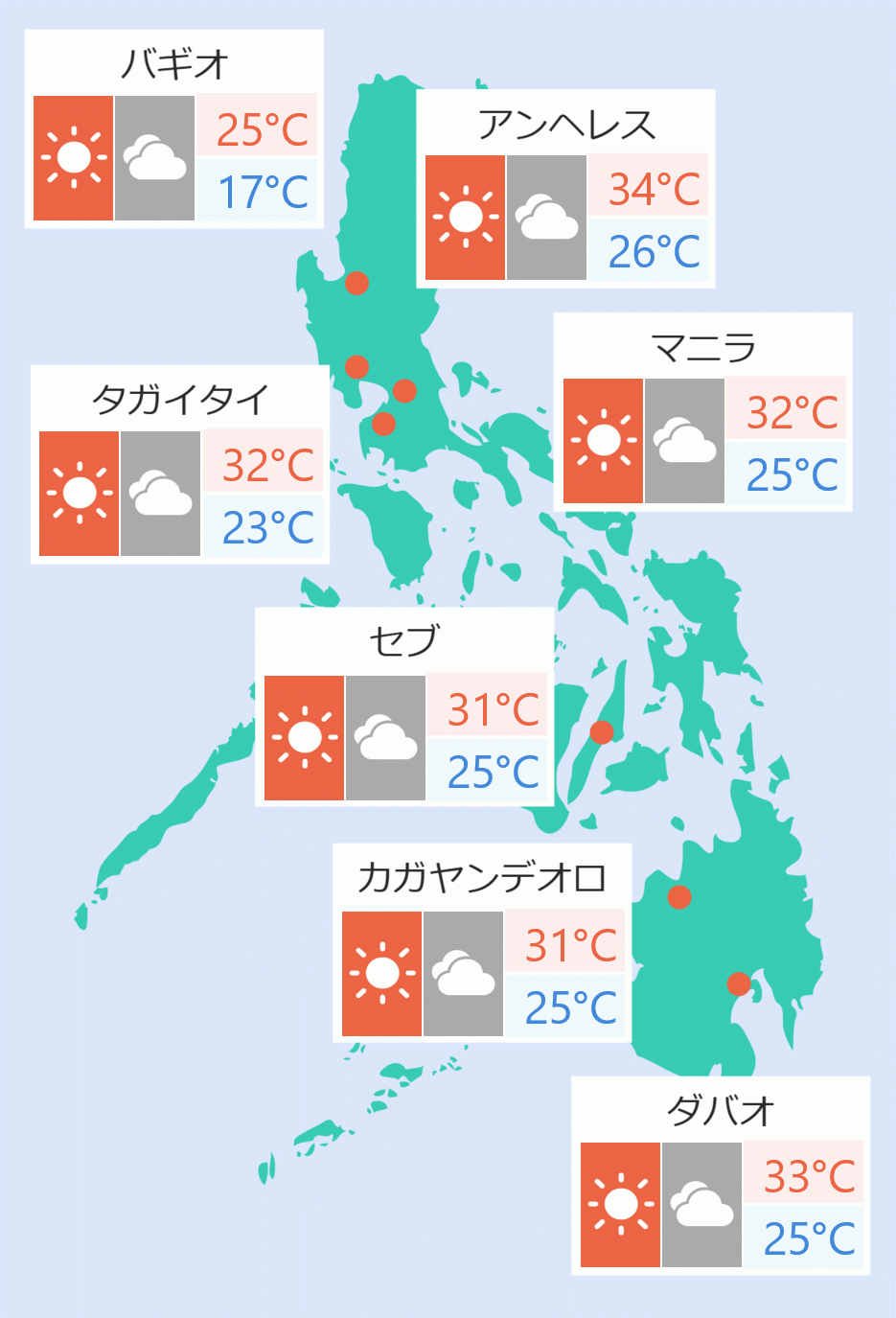
The Food and Drug Administration (FDA) said only Pfizer and Sinovac have amended its emergency use authorization (EUA) extending the shelf life of its vaccines against COVID-19.
"At this time, there is no request to extend the shelf life for AstraZeneca in particular. We have shelf life that already extends from the original six months. For example Pfizer, they have already submitted their data to show the nine months shelf life of their vaccine," FDA Director General Eric Domingo said during the ''Laging Handa'' virtual public briefing on Monday.
"Sinovac has also submitted their data to show that their shelf life is 12 months. That is why the shelf life of their vaccines are also extended," he said.
"AstraZeneca has not yet requested an amendment to their EUA to increase its shelf life from the existing six month," he added.
Domingo said the shelf lives of Pfizer and Sinovac vaccines were extended last month.
"The shelf life was extended since November, the Pfizer to nine months and the Sinovac to 12 months. When they apply for this. all the products they manufactured would be covered by the extension," he said.
In a previous interview, Health Undersecretary Myrna Cabotaje said most of the vaccines that have expired on November 30, 2021 were Astrazeneca. Robina Asido/DMS





 English
English