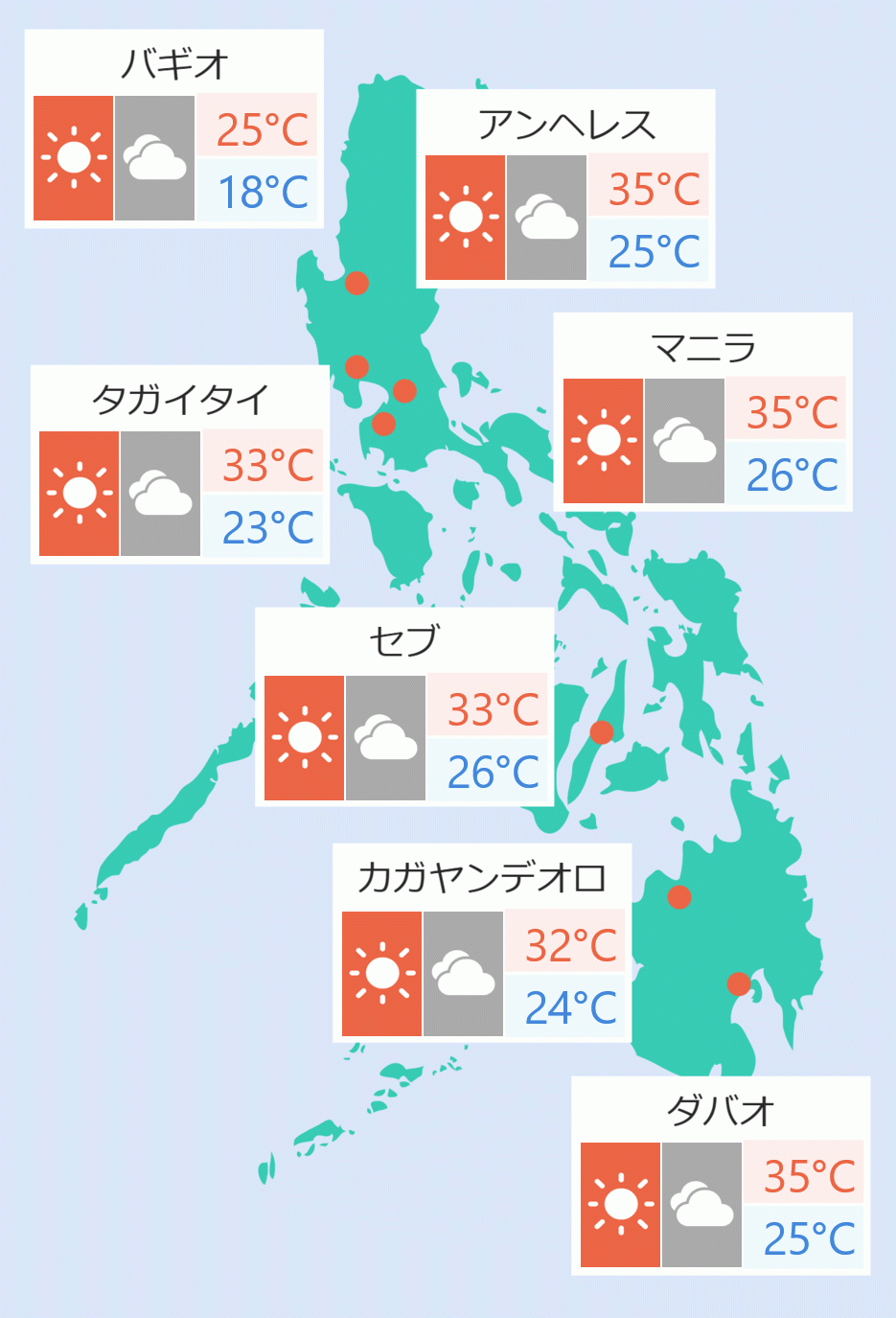
The Food and Drug Administration (FDA) granted compassionate special permits for the use of oral antiviral drug Molnupiravir, which based on clinical trials prevent death and severe COVID-19 infection, in four hospitals in the country.
In the ''Laging Handa'' public briefing Wednesday, Undersecretary Eric Domingo, FDA Director General explained that although Merck has not yet applied for emergency use authorization of Molnupiravir in the Philippines, as an investigational drug, it can apply for compassionate special permit in the country.
"In fact, as of yesterday, there are four hospitals granted with compassionate special permits for this drug by the FDA," he said.
"The clinical trial for Molnupiravir is multi-country, including the Philippines. In their interim analysis, they saw that it can prevent possibly 50 percent of people going into severe COVID and dying from COVID," he said.
Domingo said Merck will have to continue the clinical trial to apply for full registration or certificate of product registration in the country.
"They still have to continue their clinical trial because the EUA, it is being provided based on interim or like an early partial results of clinical trial. So they need to complete the clinical trial so that in the end they could get full registration or certificate of product registration, and their product can also be made available in the market," he said. Robina Asido/DMS




 English
English