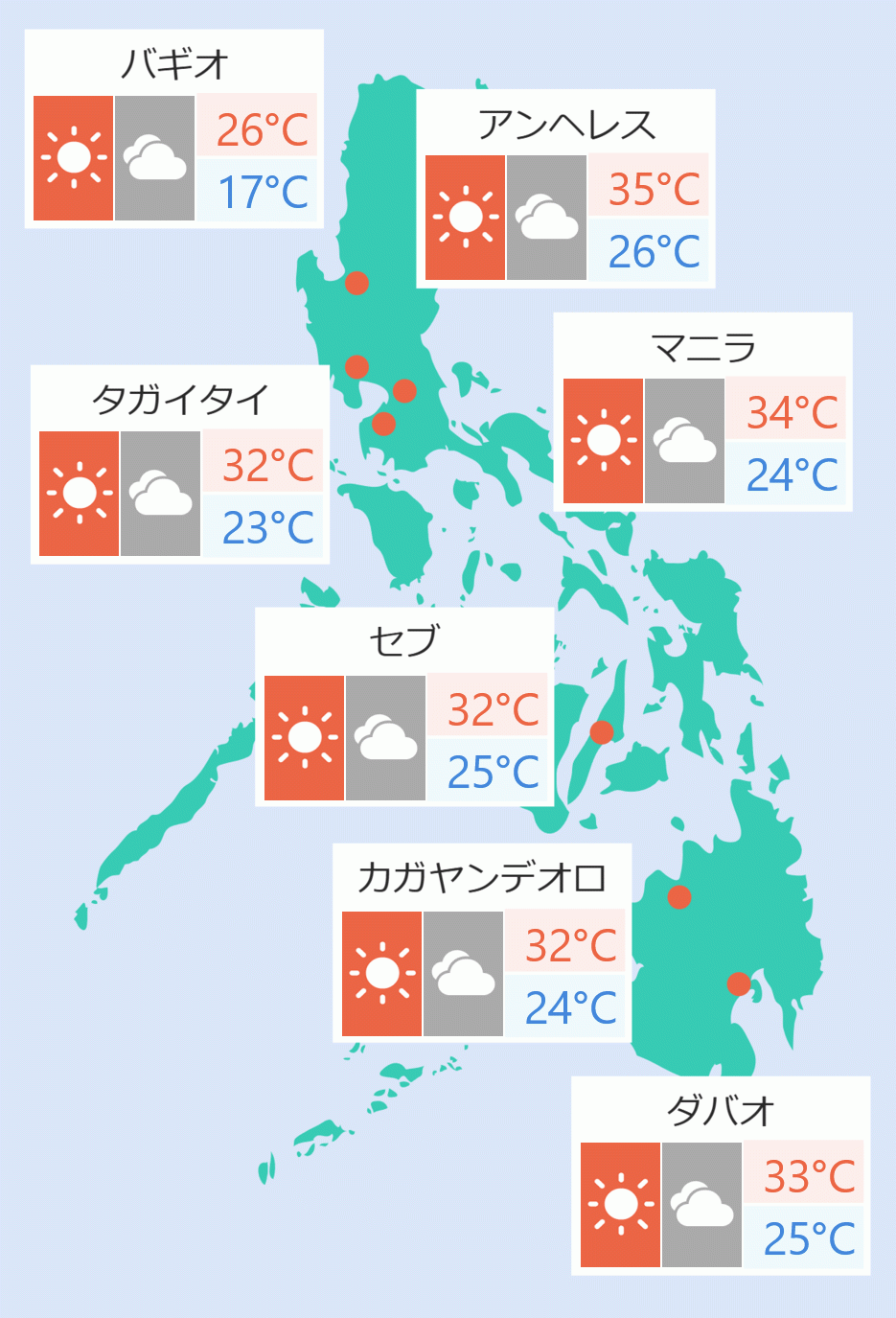
Amid the shortage of medicine used to treat severe COVID-19 patients, the Food and Drug Association (FDA) approved the use of an alternative drug manufactured in China.
"The medicine that is in demand among our people, first is Tocilizumab, we have a new equivalent product that has been approved by the FDA. It is the Tocilizumab from Livzon, it's a big biologics factory in China," FDA Director Enrique Domingo said during the taped Talk to the people on Monday night.
"Hopefully within the week the number of imported medicine will increase, so that our patients with severe Covid will be able to have access to this medicine," he added.
Domingo said the FDA also gives license for the use of two other medicines in the country similar to Baricitinib.
"The other medicine, the Baricitinib, we also have provided licence for two new similar medicine the Baricinix and Barinez. These are similar drugs, equivalent medicine that will become available here and can be used by our hospitals and can be sold to drug store to assure the supply of the medicine," he said.
Domingo also mentioned that the licensed oxygen manufacturers in the country have increased to 120.
"Since last week, there are four additional licensed oxygen manufacturers, three hospitals and one manufacturing that supplies the hospitals. So all over the country, we now have 120 licensed manufacturers of oxygen to supply our hospitals from Luzon, Visayas, and Mindanao," he said. Robina Asido/DMS





 English
English