The Philippines will be involved in the clinical trials for a vaccine against the coronavirus disease 2019( COVID-19)''by the last quarter of 2020''
In a statement Sunday, Presidential Spokesperson Harry Roque said:''We expect involvement in the vaccine clinical trials by the last quarter of 2020 with the Department of Science and Technology (DOST) taking a lead role.''
''The DOST has been tasked to provide our collaborating organizations with the Department of Health, World Health Organization and Food and Drug Administration Philippines guidelines on vaccine clinical trials; to identify the sites, the local institutions and the Filipino researchers who will be involved in the collaborative trials; to assist local participating institutions in their proposals and budgets; to obtain Ethics Board approvals; and to formalize the agreements,'' said Roque.
''President Rodrigo Roa Duterte has expressed optimism over news that clinical trials for the development of COVID-19 vaccines,'' said Roque.
''The President wants to save the life of each and every Filipino.; and thus places great interest to these clinical trials.''
In a Senate hearing on Wednesday, Health Secretary Francisco Duque III said China's biggest pharmaceutical drug company has invited the Philippines to join a global clinical trial for its vaccine.
The Philippines will be joining a similar trial for Avigan, a Japanese medicine, once Tokyo approves its use.
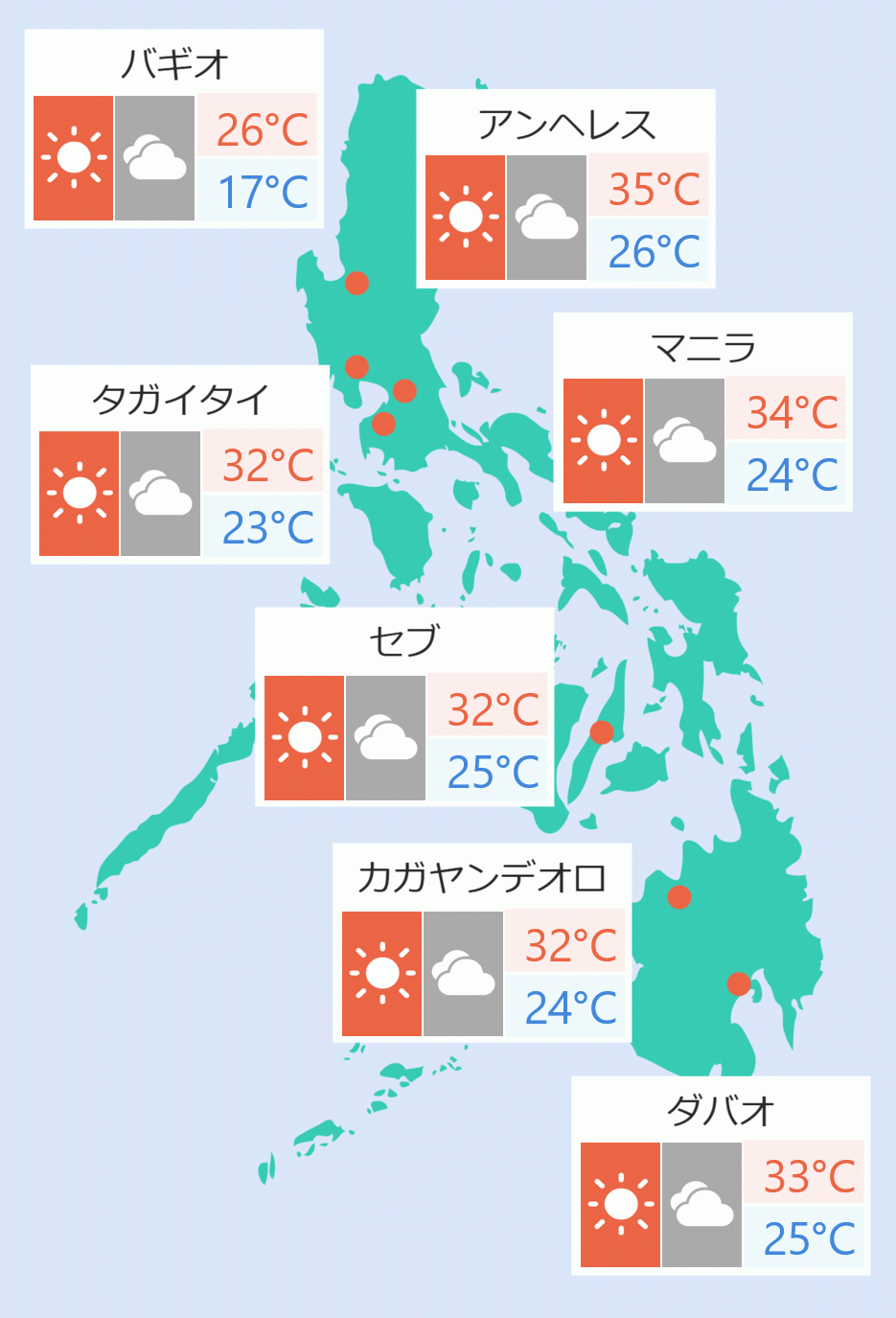
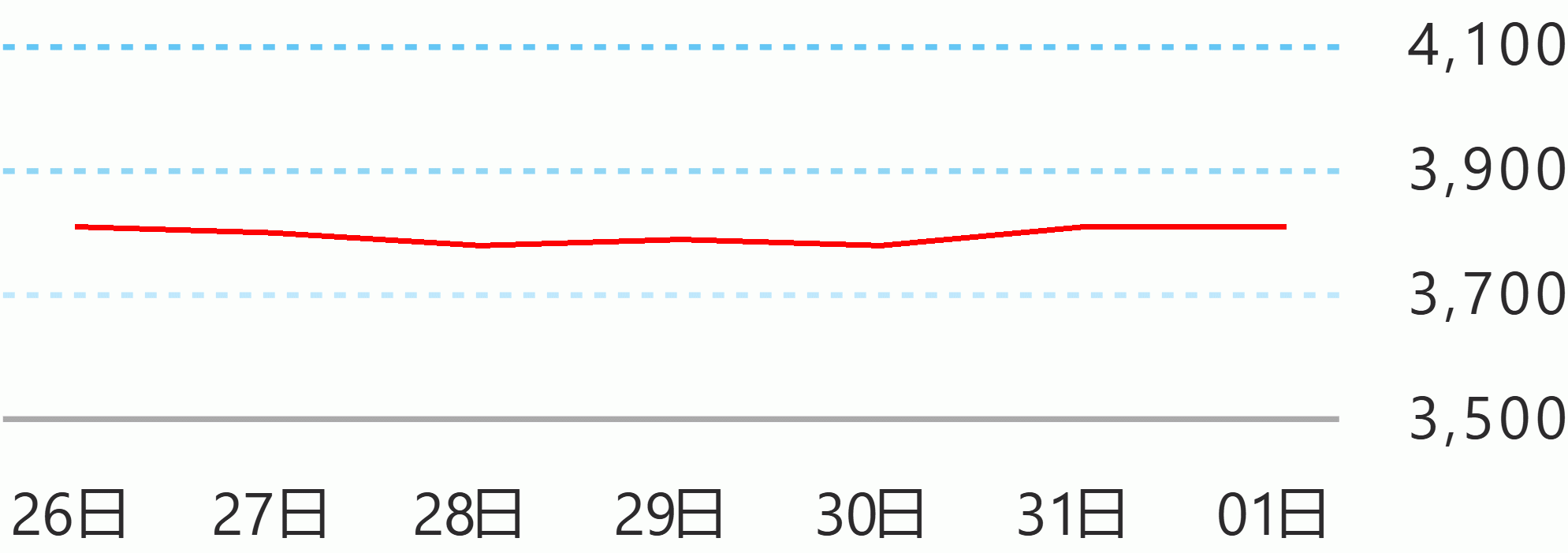
As of Sunday, the Department of Health said there were 14,035 cases, with 258 new cases. NCR accounted for 195 cases, followed by 12 from repatriates and 51 from other regions.
There were 72 recoveries bringing the total to 3,249 while five new deaths were reported, raising the tall to 868. DMS





 English
English